You are using an out of date browser. It may not display this or other websites correctly.
You should upgrade or use an alternative browser.
You should upgrade or use an alternative browser.
Coronavirus Lounge
- Thread starter JR5
- Start date

1-year risks of cancers associated with COVID-19 vaccination: a large population-based cohort study in South Korea - PubMed
The oncogenic potential of SARS-CoV-2 has been hypothetically proposed, but real-world data on COVID-19 infection and vaccination are insufficient. Therefore, this large-scale population-based retrospective study in Seoul, South Korea, aimed to estimate the cumulative incidences and subsequent...
1-year risks of cancers associated with COVID-19 vaccination: a large population-based cohort study in South Korea
The oncogenic potential of SARS-CoV-2 has been hypothetically proposed, but real-world data on COVID-19 infection and vaccination are insufficient. Therefore, this large-scale population-based retrospective study in Seoul, South Korea, aimed to estimate the cumulative incidences and subsequent risks of overall cancers 1 year after COVID-19 vaccination. Data from 8,407,849 individuals between 2021 and 2023 were obtained from the Korean National Health Insurance database. The participants were categorized into two groups based on their COVID-19 vaccination status. The risks for overall cancer were assessed using multivariable Cox proportional hazards models, and data were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). The HRs of thyroid (HR, 1.351; 95% CI, 1.206-1.514), gastric (HR, 1.335; 95% CI, 1.130-1.576), colorectal (HR, 1.283; 95% CI, 1.122-1.468), lung (HR, 1.533; 95% CI, 1.254-1.874), breast (HR, 1.197; 95% CI, 1.069-1.340), and prostate (HR, 1.687; 95% CI, 1.348-2.111) cancers significantly increased at 1 year post-vaccination. In terms of vaccine type, cDNA vaccines were associated with the increased risks of thyroid, gastric, colorectal, lung, and prostate cancers; mRNA vaccines were linked to the increased risks of thyroid, colorectal, lung, and breast cancers; and heterologous vaccination was related to the increased risks of thyroid and breast cancers. Given the observed associations between COVID-19 vaccination and cancer incidence by age, sex, and vaccine type, further research is needed to determine whether specific vaccination strategies may be optimal for populations in need of COVID-19 vaccination.
Hyperinflamation also seems to be connected to covid injections:
Hyperinflammation after anti-SARS-CoV-2 mRNA/DNA vaccines successfully treated with anakinra: Case series and literature review - PMC
The current SARS-CoV-2 pandemic diffused worldwide has encouraged the rapid development of vaccines to counter the spread of the virus. At present in Italy, 75.01% of the population completed the vaccination course (AIFA.gov.it) and very few adverse ...
They gave Anankira to some of them to cure from inflamation.
Inflamation is considered an hallmark of cancer:
Treating Cancer by Reducing Tumor-Related Inflammation
Researchers are exploring whether people with cancer benefit from treatments that reduce inflammation around tumors. Some studies show promising results.

1-year risks of cancers associated with COVID-19 vaccination: a large population-based cohort study in South Korea - PubMed
The oncogenic potential of SARS-CoV-2 has been hypothetically proposed, but real-world data on COVID-19 infection and vaccination are insufficient. Therefore, this large-scale population-based retrospective study in Seoul, South Korea, aimed to estimate the cumulative incidences and subsequent...pubmed.ncbi.nlm.nih.gov
1-year risks of cancers associated with COVID-19 vaccination: a large population-based cohort study in South Korea
The oncogenic potential of SARS-CoV-2 has been hypothetically proposed, but real-world data on COVID-19 infection and vaccination are insufficient. Therefore, this large-scale population-based retrospective study in Seoul, South Korea, aimed to estimate the cumulative incidences and subsequent risks of overall cancers 1 year after COVID-19 vaccination. Data from 8,407,849 individuals between 2021 and 2023 were obtained from the Korean National Health Insurance database. The participants were categorized into two groups based on their COVID-19 vaccination status. The risks for overall cancer were assessed using multivariable Cox proportional hazards models, and data were expressed as hazard ratios (HRs) and 95% confidence intervals (CIs). The HRs of thyroid (HR, 1.351; 95% CI, 1.206-1.514), gastric (HR, 1.335; 95% CI, 1.130-1.576), colorectal (HR, 1.283; 95% CI, 1.122-1.468), lung (HR, 1.533; 95% CI, 1.254-1.874), breast (HR, 1.197; 95% CI, 1.069-1.340), and prostate (HR, 1.687; 95% CI, 1.348-2.111) cancers significantly increased at 1 year post-vaccination. In terms of vaccine type, cDNA vaccines were associated with the increased risks of thyroid, gastric, colorectal, lung, and prostate cancers; mRNA vaccines were linked to the increased risks of thyroid, colorectal, lung, and breast cancers; and heterologous vaccination was related to the increased risks of thyroid and breast cancers. Given the observed associations between COVID-19 vaccination and cancer incidence by age, sex, and vaccine type, further research is needed to determine whether specific vaccination strategies may be optimal for populations in need of COVID-19 vaccination.
Hyperinflamation also seems to be connected to covid injections:
Hyperinflammation after anti-SARS-CoV-2 mRNA/DNA vaccines successfully treated with anakinra: Case series and literature review - PMC
The current SARS-CoV-2 pandemic diffused worldwide has encouraged the rapid development of vaccines to counter the spread of the virus. At present in Italy, 75.01% of the population completed the vaccination course (AIFA.gov.it) and very few adverse ...pmc.ncbi.nlm.nih.gov
They gave Anankira to some of them to cure from inflamation.
Inflamation is considered an hallmark of cancer:

Treating Cancer by Reducing Tumor-Related Inflammation
Researchers are exploring whether people with cancer benefit from treatments that reduce inflammation around tumors. Some studies show promising results.www.cancer.gov


Normies being heavily gaslit.
What do you mean? The experimental mutagens clearly increase cancer survival rates, a timely blessing as cancer rates have risen since their introduction.
"I know a sonographer who's been a sonographer for 40 years, and she reads ultrasounds. So she can read ultrasounds, and she can tell the mother, 'Oh, you got Pfizer, you got Moderna.' And if they're like, well, no, I didn't get the vaccine. Well, the placenta has these characteristics of this shot, and the dad is responsible for the, placenta health. And so the placenta will carry dad's DNA. So, if it's there, it's from dad. So even if the mother didn't receive the shot, but the dad did, it still will affect the baby... That mRNA Protein. It's in the sperm also...
"[And] the babies are still being born dead, and we're in 2025. And so mothers aren't necessarily receiving the COVID vaccines anymore. They are getting cov— They're getting vaccinated for TDAP, the flu. And then, like, RSV is a newer one. When they go to their doctor, they push all of these. So whether it's Covid or it's these new ones, I— I'm not sure. But the babies are still dying. And it's. Every time I go to work, there's a dead baby. And that, never should be normal.
"And I have asked many nurses— Are they okay with this? Yep. And they all say, I appreciate what you're doing. I believe you, but I can't lose my job."
Never forget
Never forget
I still get asked if I have "cold or cough symptoms" when visiting the doctors here in luny kangaroo land.
My favorite was when they said, "Covid has no symptoms"I still get asked if I have "cold or cough symptoms" when visiting the doctors here in luny kangaroo land.
Vaccine Stocks Drop After FDA Memo Links COVID Shots To Child Deaths
Vaccine Stocks Drop After FDA Memo Links COVID Shots To Child Deaths | ZeroHedge
ZeroHedge - On a long enough timeline, the survival rate for everyone drops to zero
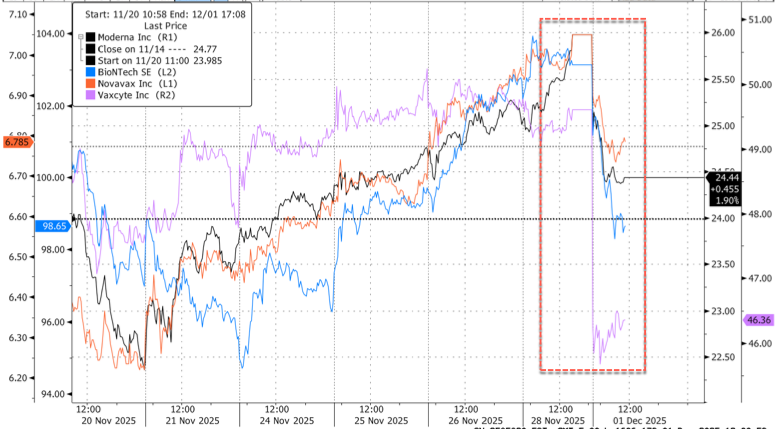
Vaccine stocks slumped Monday after an explosive memo from FDA vaccine chief Vinay Prasad surfaced late Friday, signaling the agency is preparing to roll out tough restrictions on new vaccines for children. Prasad described a "profound revelation" linking Covid shots to at least ten deaths in children.
By late morning, Vaccine makers dropped on the memo: Moderna -6%, BioNTech -4.3%, Novavax -4%, Vaxcyte -6.6%.
"This is a profound revelation," Prasad wrote in the memo. "For the first time, the US FDA will acknowledge that COVID-19 vaccines have killed American children."
He added, "It is horrifying to consider that the US vaccine regulation, including our actions, may have harmed more children than we saved. This requires humility and introspection."
Wall Street analysts weighed in on the memo, and all agreed it introduces a new regulatory overhang for vaccine stocks.
Here's what the research desks told clients:
The memo comes months after the Trump administration signaled it would link Covid shots to children's deaths. Remember, anyone who questioned the vaccines in the early days of the pandemic was demonized by Democrats and "trust the science" regime, which unleashed big-tech and state-sponsored censorship cartel against anyone asking questions.William Blair, Myles R. Minter (rates the MRNA market perform)
Mizuho, Salim Syed (rates PCVX outperform)
- "Our interpretation of the memo is that CBER will focus its efforts on the younger 12- to 24-year-old male population for newly approved Covid-19 vaccines where the myocarditis risk is highest"
- If new regulatory restrictions were to be implemented in the higher myocarditis risk population, analysts see further headwinds toward Moderna's declining Covid-19 franchise "alongside further negative sentiment that this memo and subsequent actions may generate"
- Analyst says Pfizer, BioNTech, Novavax and Sanofi could also be impacted
- "The memo also indicates several upcoming reforms to the CBER vaccine regulatory pathway, most notably the "demand" for pre- market randomized trials assessing clinical endpoints, not just immunogenicity, for most new vaccine products"
Cantor, Carter Gould (rates PCVX overweight)
- Says the memo notes "pneumonia vaccine makers will have to show their products reduce pneumonia (at least in the post- market setting), and not merely generate antibody titers"
- However, "what investors are missing here is this is already in-line with the current standard" and poses no material change to Vaxcyte
Leerink Partners, Mani Foroohar (rates MRNA underperform)
- Says not surprised to see selloff in PCVX shares "on the back of the return of perceived regulatory risk after a period of relative calm, particularly with key data weighted to late 2026"
- However, analyst says there wasn't much in the actual memo language on pneumococcal vaccines (PCVs) that's concerning
- Reminds investors that "this all needs to continue to be viewed in the context of the likely timelines for VAX-31 adult and infants efforts against the backdrop of the time remaining in the current administration's term"
- "We appreciate that there's plenty within the memo that's controversial or worrisome regarding Covid-19 vaccine policy, but the actual language on PCVs shows little evolution vs. prior guidance"
- Says the memo's inflammatory tone highlights how agency policy/communications continue to contribute to vaccine skepticism and US vaccination rate decline
- "We view this as a continued negative for mRNA vaccine manufacturers in our coverage– especially as it relates to Moderna's recently updated short-to-mid-term revenue guidance"

Supreme Court Intensifies Review of Vaccine Mandates | America's Frontline Doctors
Medical And Policy Resources To Defend Your Freedom ... Advocates for Liberty, Health, and Justice. Join Us.…
Supreme Court Intensifies Review of Vaccine Mandates
Key Orders Elevate Religious Liberty and Constitutional LimitsWashington, DC | December 8, 2025
America’s Frontline Doctors (AFLDS) announced today two consequential actions by the U.S. Supreme Court that sharply escalate judicial scrutiny of coercive vaccine mandates and reaffirm that constitutional limits remain fully operative in times of crisis.
RIP Scott Adams, who mocked "vaccine deniers" during the "pandemic".

'Dilbert' creator Scott Adams dies at 68 after prostate cancer battle
Scott Adams, the cartoonist whose "Dilbert" comic strip satirized corporate life before racist comments he made sidelined him, has died at 68.
www.usatoday.com